
Sample type:
Delivery period:
Acetylsalicylic acid (Aspirin®) DNA test
Treatment with ASA in primary prevention of cardiovascular disease is not indicated because the risk of hemorrhage is higher than that of cardiovascular events that are avoided.
However, having a variant in the Lp (a) gene confers a higher risk of suffering a cardiovascular event, thus secondary prevention, that is, there is no obvious clinical risk, but there is a genetic risk that justifies it.
The FDA-USA in a May 2, 2014 release, "Use of Aspirin for Primary Prevention of Heart Attack and Stroke," says that it does not consider that there is evidence to support the general use of AAS for the primary prevention of a heart attack or stroke.
In fact, according to the FDA's consideration, there are serious risks associated with the use of ASA, including an increased risk of bleeding in the stomach and brain, in situations where the benefit of ASA for primary prevention has not been established.
It has been shown that the risk of side effects, mainly gastrointestinal or even brain hemorrhages, is greater than the number of possible cardiovascular events, which are intended to prevent. Primary prevention of a disease is to take preventive actions, when there are no symptoms, no alteration or no risk factors found in medical examinations of the patient.
Secondary prevention: It is when the patient has no symptoms, but there are risk factors in medical examinations or a history of disease.
AAS administered in a preventive way is indicated in secondary prevention, which is, when the patient has positive symptoms or cardiovascular risk indicators, or has already had an incident of thrombosis. Preventive ASA is NOT indicated in primary prevention.
Interpretation of the report in polytherapy
In this report, the "green" labeling of a certain drug with the patient's genes indicates that it can be prescribed in the standard dose conditions disclosed in the prospectus or those recommended by the physician. This applies in the case of monotherapy.
However, if the patient takes several medications, which is very common, although in the report would appear in "green" can cause adverse effects due to possible interactions between them, which can sometimes be severe, either by toxicity or by therapeutic failure.
Drugs are chemicals that may interfere the normal metabolism of other drugs. Interferences that are also applicable to the chemical substances of various medicinal plants and food supplements.
Consequently, the whole of the medication, together with the consumer products or life habits of the patient, must undergo an evaluation of pharmacological interactions.
Consulting g-Nomic® Pharmacogenetics interpretation software, before prescribing, is an effective aid to avoid most adverse effects or therapeutic failures due to medication.
g-Nomic® interrelates all drugs, herbs, nutritional supplements and lifestyle, with the patient's genes. It also advises the most appropriate dose to prescribe.
This text has been translated automatically using an electronic translator, so the Spanish version should prevail. Please check in case of doubt.
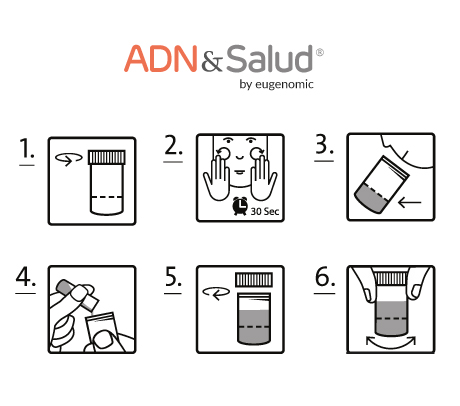
You will receive the sample collection kit. You can request additional kits if you need it.
Sample collection kit content:
You will get a box with sample collection kit. Within the kit you will find:
- Saliva collector.
- Instructions for the sample collection.
- Required documents
- Security bag to enter the sample for shipment.
- Envelope bag.
Process to follow:
- Fill in the documents that we will send you, duly signed. You can also find these documents on this page, under the web tab: "Documents required".
- Follow the instructions for collecting the sample.
- After obtaining the sample, include it in the return envelope remember to put into the necessary documents.
- Call Eugenomic® on +34 93 292 29 63 or email info@eugenomic.com to request sample collection. No shipping costs on Spanish territory.
- An email confirmation will be sent when your sample si received.
- When the study will be completed you will receive a new e-mail to inform you that your report is already available in "My results"
Info producto mobile
Profile Information
Acetylsalicylic acid (Aspirin®) DNA test
Treatment with ASA in primary prevention of cardiovascular disease is not indicated because the risk of hemorrhage is higher than that of cardiovascular events that are avoided.
However, having a variant in the Lp (a) gene confers a higher risk of suffering a cardiovascular event, thus secondary prevention, that is, there is no obvious clinical risk, but there is a genetic risk that justifies it.
The FDA-USA in a May 2, 2014 release, "Use of Aspirin for Primary Prevention of Heart Attack and Stroke," says that it does not consider that there is evidence to support the general use of AAS for the primary prevention of a heart attack or stroke.
In fact, according to the FDA's consideration, there are serious risks associated with the use of ASA, including an increased risk of bleeding in the stomach and brain, in situations where the benefit of ASA for primary prevention has not been established.
It has been shown that the risk of side effects, mainly gastrointestinal or even brain hemorrhages, is greater than the number of possible cardiovascular events, which are intended to prevent. Primary prevention of a disease is to take preventive actions, when there are no symptoms, no alteration or no risk factors found in medical examinations of the patient.
Secondary prevention: It is when the patient has no symptoms, but there are risk factors in medical examinations or a history of disease.
AAS administered in a preventive way is indicated in secondary prevention, which is, when the patient has positive symptoms or cardiovascular risk indicators, or has already had an incident of thrombosis. Preventive ASA is NOT indicated in primary prevention.
Interpretation of the report in polytherapy
In this report, the "green" labeling of a certain drug with the patient's genes indicates that it can be prescribed in the standard dose conditions disclosed in the prospectus or those recommended by the physician. This applies in the case of monotherapy.
However, if the patient takes several medications, which is very common, although in the report would appear in "green" can cause adverse effects due to possible interactions between them, which can sometimes be severe, either by toxicity or by therapeutic failure.
Drugs are chemicals that may interfere the normal metabolism of other drugs. Interferences that are also applicable to the chemical substances of various medicinal plants and food supplements.
Consequently, the whole of the medication, together with the consumer products or life habits of the patient, must undergo an evaluation of pharmacological interactions.
Consulting g-Nomic® Pharmacogenetics interpretation software, before prescribing, is an effective aid to avoid most adverse effects or therapeutic failures due to medication.
g-Nomic® interrelates all drugs, herbs, nutritional supplements and lifestyle, with the patient's genes. It also advises the most appropriate dose to prescribe.
This text has been translated automatically using an electronic translator, so the Spanish version should prevail. Please check in case of doubt.
Sample type and process to follow

You will receive the sample collection kit. You can request additional kits if you need it.
Sample collection kit content:
You will get a box with sample collection kit. Within the kit you will find:
- Saliva collector.
- Instructions for the sample collection.
- Required documents
- Security bag to enter the sample for shipment.
- Envelope bag.
Process to follow:
- Fill in the documents that we will send you, duly signed. You can also find these documents on this page, under the web tab: "Documents required".
- Follow the instructions for collecting the sample.
- After obtaining the sample, include it in the return envelope remember to put into the necessary documents.
- Call Eugenomic® on +34 93 292 29 63 or email info@eugenomic.com to request sample collection. No shipping costs on Spanish territory.
- An email confirmation will be sent when your sample si received.
- When the study will be completed you will receive a new e-mail to inform you that your report is already available in "My results"

